Pharmaceuticals
Trusted Logistical Transport Solutions
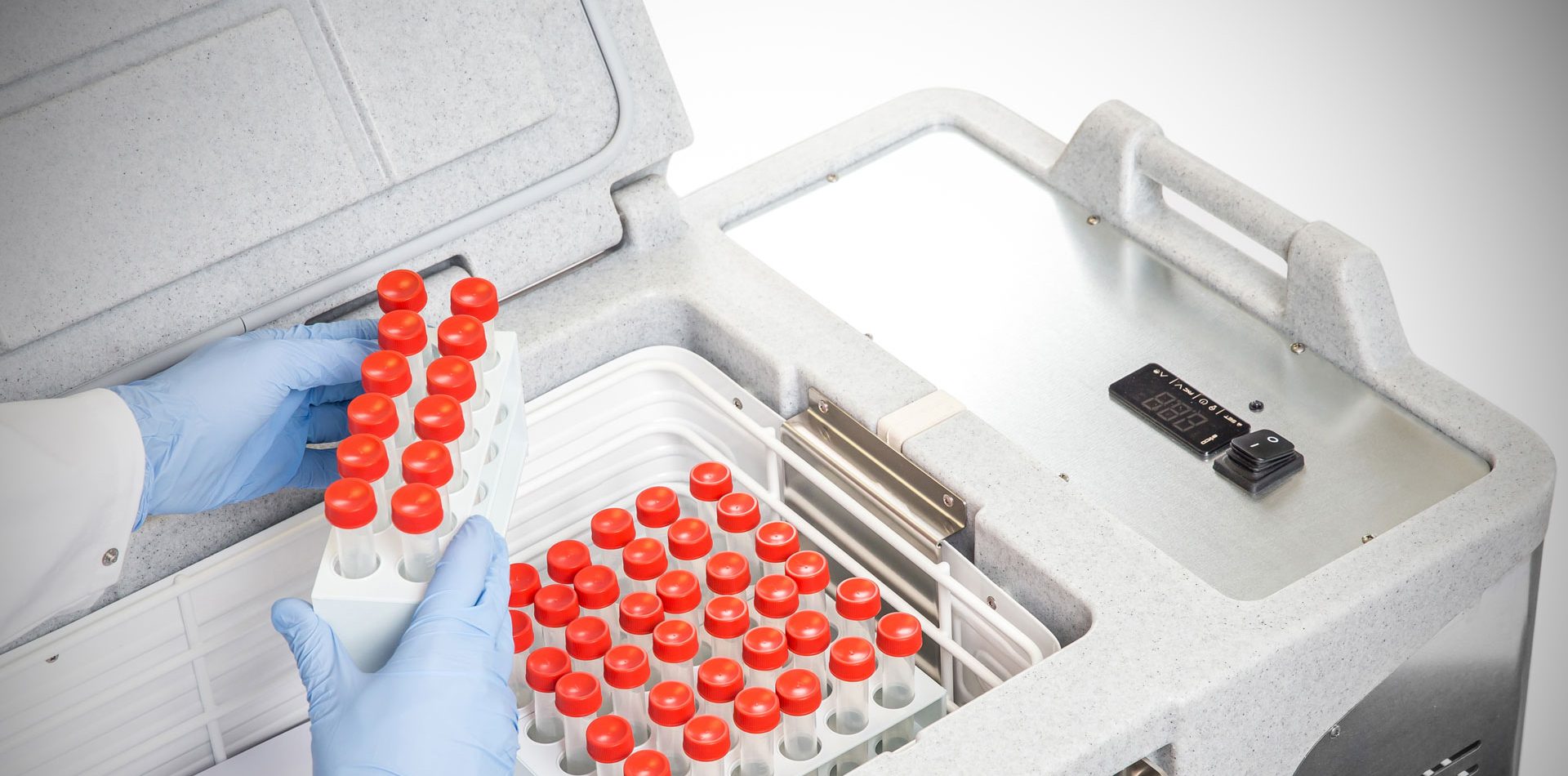
Hospitals, labs, research centres and military facilities around the world depend on Coldtainer’s refrigerated medical transport containers to safely and securely transport medical materials and pharmaceutical products. Medical manufacturers and distribution networks must meet stringent requirements in order pass the guidelines imposed by regulatory health and drug agencies. Maintaining the integrity and precise temperatures of medical and pharmaceutical products during their transport is key to preserving their efficacy.