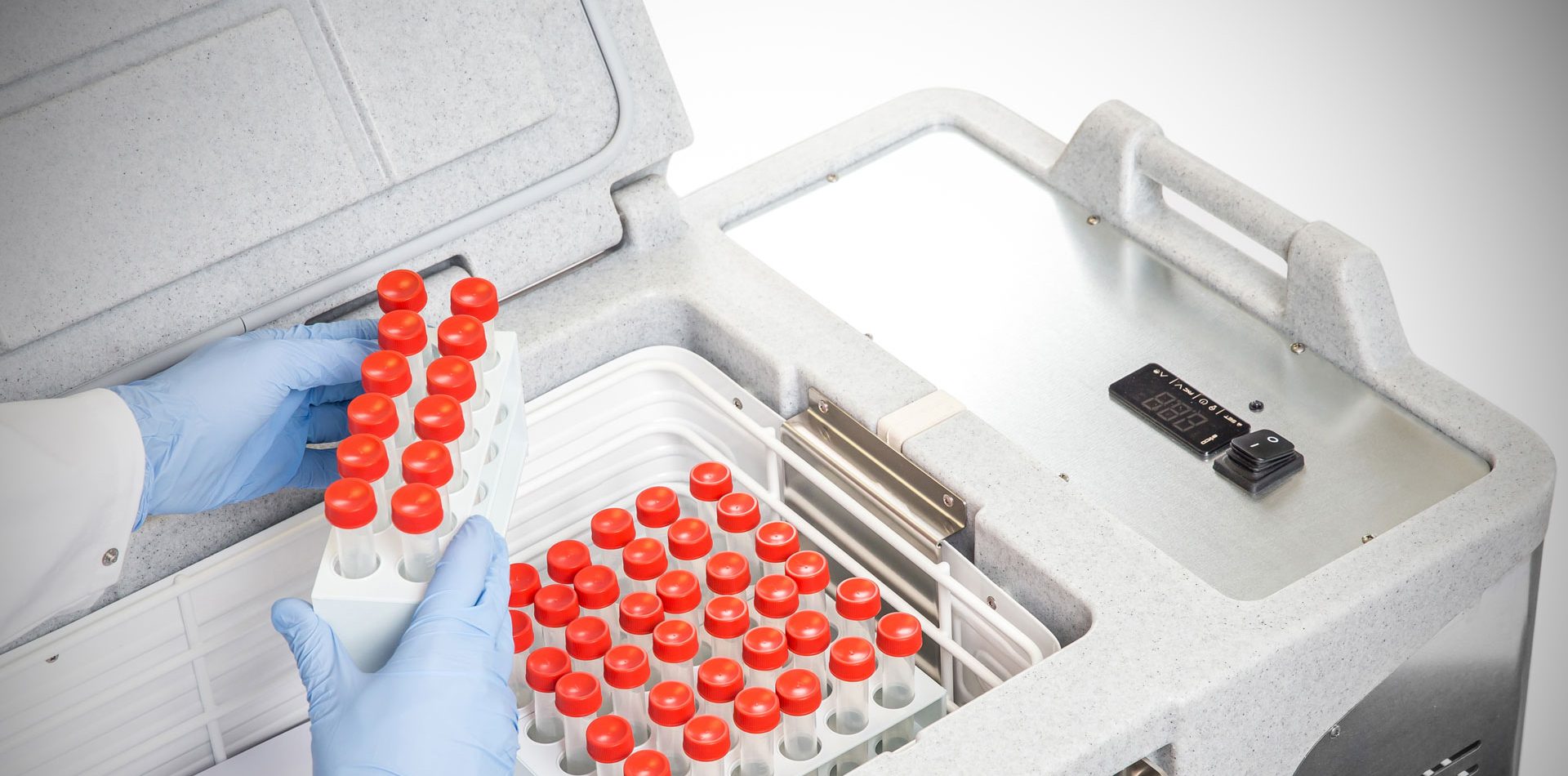
When Compliance is Critical
PORTABLE
FLEXIBLE
100% GREEN
Considerations for Refrigerated Medical Transport
Coldtainer’s complete line of portable temperature controlled containers are used across a diverse range of industries including medical and pharmaceutical transport and as a result are not classified as “Medical Devices.” For this reason, Coldtainer units are not required to meet the standards or authorizations for such devices. When using Coldtainer’s refrigerated medical transport containers to transport UN 3373 biological substances, you must follow the required system packaging for the product being shipped as indicated by shipping services provider.
Medical and Military Approved
- Pharmaceutical-distribution companies
- Medical Military and civil protection bodies
- Research laboratories and universities
- Public-health authorities
Coldtainers are double-walled with rounded corners (and without joints) allowing for HACCP-level cleaning. They are light, rugged, and manufactured using rotational molding technology that guarantees performance and durability even in the presence of constant vibrations.
Without the need for dedicated transport infrastructure and insulation, these containers provide the flexibility to easily load, unload, and stack in a variety of configurations depending on your delivery requirements. Contact us today to see how Coldtainer can help your pharmaceutical business.
Coldtainer’s portable temperature controlled containers are used across a diverse range of industries including medical and pharmaceutical transport, as well as research laboratories, universities, public health authorities, and medical military and civil protection bodies.
Coldtainer’s refrigerated medical transport containers have an ambient temperature range of -20°C up to +50°C (with reduced specifications). Specific Coldtainer models also offer XFDN versions that maintain temperatures down to -30°, with automatic on-board heating and cooling systems that normalize for external environmental temperature conditions in compliance with applicable international regulations.
No, Coldtainer’s complete line of portable temperature controlled containers are not classified as “Medical Devices,” and as a result are not required to meet the standards or authorizations for such devices.
When using Coldtainer’s refrigerated medical transport containers to transport UN 3373 biological substances, you must follow the required system packaging for the product being shipped as indicated by the shipping services provider.
Coldtainer’s refrigerated medical transport containers are double-walled with rounded corners and no internal joints and edges, allowing for HACCP-level cleaning.

CONFIGURE YOUR STORAGE SOLUTION
Let us help identify the portable temperature-sensitive storage possibilities for your business.